Abstract
Background: BTK inhibitors and BCL2 antagonists have shown (separately) to be safe and effective in patients with Waldenström macroglobulinemia (WM). We, therefore, designed a prospective study evaluating the combination of the BTK inhibitor ibrutinib and the BCL2 antagonist venetoclax in previously untreated patients with WM.
Aims: To evaluate the safety and efficacy of the combination of ibrutinib and venetoclax in previously untreated patients with WM.
Methods: This was an investigator-initiated, single-arm, multicenter, prospective phase II study. Assuming a null VGPR rate of <25% and an alternative VGPR rate of >45%, a sample size of 50 patients was needed (power 80%, alpha 3%). All patients met diagnostic criteria for WM, met criteria to treat, and provided written consent. Study therapy was given in 4-week cycles. Ibrutinib was started at a dose of 420 mg orally daily starting on cycle 1. On cycle 2, venetoclax was started at 100 mg orally daily for 1 week, then 200 mg orally daily for 1 week, and then 400 mg orally daily for 2 weeks. Both agents were given concurrently starting on cycle 3 to complete 24 cycles. The patients were monitored for tumor lysis syndrome monitoring during cycle 2 (venetoclax ramp up). All patients underwent baseline laboratory studies, a bone marrow biopsy for MYD88 and CXCR4 genotyping, and computed tomography scans to evaluate for extramedullary disease. Responses were assessed using modified criteria by the 6th International Workshop for WM. Our Institutional Review Board approved the present study, registered under ClinicalTrials.Gov ID NCT04273139.
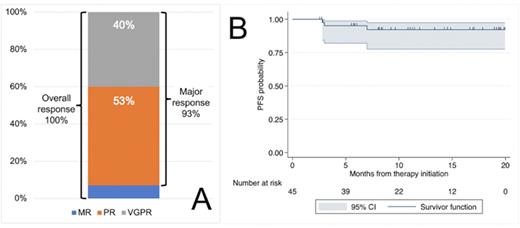
Results: Between July 2020 and January 2022, a total of 45 patients were enrolled. Baseline characteristics were: median age 67 years (range 40-82), male:female ratio was 2:1 (30:15), MYD88 L265P 45 (100%), CXCR4 mutation 17 (38%), serum IgM 4297 mg/dl (range 572-9211), hemoglobin 10.2 g/dl (range 7.8-15.3), bone marrow involvement 60% (5-90%), lymphadenopathy >1.5 cm 25 (56%), splenomegaly >15 cm 13 (29%), von Willebrand disease 9 (20%), and cryoglobulins 3 (7%). Best categorical responses were VGPR 18 (40%), partial response (PR) 24 (53%), and minor response (MR) 3 (7%), for an ORR of 100% and a major response rate of 93% (Figure A). The median time to MR was 1.9 months, without difference in CXCR4 mutated patients (p=0.27), and the median time to major response was 1.9 months and was longer in CXCR4 mutated patients (2.8 v 1.8; p=0.048). The VGPR rate was lower in CXCR4 mutated patients (24% v 50%; p=0.08). There was improvement in lymphadenopathy in 82% and in splenomegaly in 92% of patients. With a median follow-up of 11 months, 1 patient progressed with transformation to aggressive lymphoma, and 2 patients died of ventricular arrhythmia. The estimated 12-month PFS rate is 92% (Figure B). Grade ≥3 adverse events seen in >1 patient included neutropenia (n=13; 29%), cardiac arrest/ventricular arrhythmia (n=3; 7%), oral mucositis (n=2; 4%), and tumor lysis syndrome (n=2; 4%). On January 19, 2022, a third Grade ≥4 cardiac arrest/ventricular arrhythmia was reported prompting a hold on further accrual given the higher-than-expected rate of events. All participants on active treatment were required to undergo cardiac evaluations including ECGs, echocardiograms, and stress tests. On April 6, 2022, one participant developed a Grade 2 ventricular tachycardia while undergoing a stress test, prompting a full clinical hold on this trial given a rate of ventricular arrhythmia/cardiac arrest of 9% (n=4). All active participants stopped study therapy and are under active follow-up.
Conclusion: The combination of ibrutinib and venetoclax was associated with a major response rate of 93%, and a VGPR rate of 40% in previously untreated WM. However, there was a higher-than-expected rate of ventricular arrhythmia of 9%, which prompted stopping study therapy.
Disclosures
Castillo:TG Therapeutics: Research Funding; Roche: Consultancy; Janssen: Consultancy; Cellectar: Consultancy; Beigene: Consultancy, Research Funding; AstraZeneca: Research Funding; Pharmacyclics: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding. Sarosiek:ADC Therapeutics: Research Funding; BeiGene: Consultancy. Branagan:Janssen/Pharmacyclics: Consultancy; Adaptive Biotechnologies: Consultancy; Genzyme: Consultancy; BeiGene: Consultancy; CSL Behring: Consultancy; Karyopharm Therapeutics: Consultancy. Treon:Abbvie: Consultancy; Janssen/Pharmacyclics: Consultancy, Research Funding; BMS: Research Funding; BeiGene: Consultancy; X4: Consultancy, Research Funding.
OffLabel Disclosure:
Venetoclax is not approved for the frontline treatment of Waldenström macroglobulinemia.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal